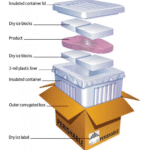
O transporte de vacinas exige controle rigoroso de temperatura para manter sua eficácia. A faixa de 2 a 8 ° C é crucial, Como mesmo pequenas flutuações de temperatura podem comprometer sua eficácia. Então, how can Sacos de gelo seco keep vaccines within this critical range? Gelo seco, typically used for ultra-cold storage, requires careful management to ensure it doesn’t freeze the vaccines. This article delves into how dry ice bags, when used with the right technology, can maintain vaccines within the 2–8°C range during transit.
-
Dry ice can help transport vaccines at the 2–8°C range when used with phase-change materials (PCMs) and proper insulation.
-
Temperature monitoring systems are essential for ensuring the cold chain remains intact during transit.
-
Real-time tracking of vaccine shipments is a growing trend in cold chain logistics.
-
Sustainable and eco-friendly materials are increasingly used in dry ice bags to reduce environmental impact.
Can Dry Ice Bags Keep Vaccines Within the 2–8°C Range?
The simple answer is yes, but with some key caveats. Dry ice sublimates at -78.5°C, which makes it unsuitable for directly maintaining a 2–8°C range. No entanto, dry ice can be used effectively for vaccine transport when combined with insulated containers and phase-change materials (PCMs). PCMs help regulate the temperature by absorbing or releasing heat as they change phases, ensuring that the temperature remains stable within the desired range.
Core Points:
-
Isolamento adequado: Insulated containers designed for pharmaceutical transport help minimize temperature fluctuations and protect vaccines from external heat sources.
-
PCMs for Temperature Stability: PCMs are pre-conditioned to maintain the 2–8°C range. When used alongside dry ice, they buffer any temperature fluctuations, ensuring the vaccines remain safe.
-
Real-time Monitoring: Data loggers and temperature sensors are essential tools for tracking the temperature during transport. Alerts can notify the handlers if the temperature goes beyond the acceptable range, allowing for quick corrective actions.
How Does Dry Ice Work in Vaccine Transport?
Dry ice helps maintain low temperatures, but the cold it generates must be carefully managed. When used in shipping, dry ice sublimes, converting from a solid to a gas. This process can keep the vaccines cool, but without proper insulation and temperature regulation, it could potentially freeze them.
Key Practices for Using Dry Ice Bags
-
Pre-conditioning PCMs: Before packing, ensure that PCMs are conditioned to the 2–8°C range to regulate the temperature efficiently during transit.
-
Monitoramento de temperatura: Continuous temperature tracking with data loggers ensures the cold chain stays intact and any deviations are immediately addressed.
-
Compliance with Health Guidelines: Transporting vaccines requires adherence to strict guidelines from health organizations such as the CDC and WHO to ensure the vaccines remain effective.
Practical Applications and Case Studies
Estudo de caso 1: COVID-19 Vaccine Distribution
Durante a pandemia de COVID-19, dry ice was used in combination with insulated packaging and PCMs to maintain the required temperature for vaccines, ensuring their effectiveness throughout transit.
Estudo de caso 2: Routine Vaccine Deliveries
For routine vaccine deliveries, dry ice bags combined with PCMs have proven effective in maintaining the 2–8°C range, even in remote locations with limited access to refrigeration.
What Factors Impact the Effectiveness of Dry Ice in Vaccine Transport?
Dry ice is not a one-size-fits-all solution. The effectiveness of dry ice in transporting vaccines depends on several factors:
-
Quantidade de gelo seco: Using more dry ice can extend the cooling duration. No entanto, too much dry ice can cause temperatures to fall below the safe range, potentially freezing the vaccines.
-
Qualidade de isolamento: High-quality insulated containers, such as those with thermal blankets or foam insulation, are critical for regulating temperature.
-
External Environmental Conditions: External temperature and humidity can affect the sublimation rate of dry ice, requiring adjustments to the amount used.
Exemplo do mundo real:
A pharmaceutical company successfully transported vaccines using dry ice bags, PCMs, and insulated packaging, achieving a stable 2–8°C temperature range for over 72 hours despite extreme environmental conditions.
Key Factors for Successful Dry Ice Vaccine Transport
| Fator | Práticas recomendadas | Impact on Transport |
|---|---|---|
| Quantidade de gelo seco | Ensure optimal amount based on shipment duration | Controls how long the cooling effect lasts |
| Qualidade de isolamento | Use insulated containers with phase-change materials | Ensures stable temperature and prevents freezing |
| External Conditions | Monitor and adjust based on climate | Prevents excessive sublimation and temperature deviations |
How to Control Dry Ice Usage for Vaccine Transport?
To ensure vaccines stay within the 2–8°C range, it’s important to control the amount of dry ice used:
-
Estimate Shipment Duration: Shorter trips require less dry ice, while longer trips need more to compensate for sublimation loss.
-
Monitore as taxas de sublimação: Track how quickly the dry ice sublimates using data loggers, adjusting as needed based on environmental factors.
Temperature Monitoring for Safe Vaccine Transport
Temperature monitoring is essential to ensure vaccine safety. Even with the best packaging, there can be subtle fluctuations that compromise the vaccines. Using data loggers or smart sensors, the temperature can be continuously tracked throughout the transport process, with real-time alerts sent if the temperature goes outside the safe range.
Essential Tools:
-
Registradores de temperatura: Track the temperature inside the package to ensure the cold chain remains intact.
-
Sensores inteligentes: Provide instant notifications if the temperature fluctuates beyond the required range.
Future Trends in Vaccine Transport Solutions
Em 2025, the focus on cold chain logistics continues to evolve, with new technologies enhancing the efficiency and sustainability of vaccine transport.
Latest Developments in Cold Chain Logistics:
-
Materiais de Embalagem Sustentáveis: There’s a push towards biodegradable PCMs and eco-friendly packaging materials to reduce the environmental impact of vaccine transport.
-
Tecnologias avançadas de isolamento: New innovations in insulation materials are making it easier to maintain stable temperatures while using less dry ice.
-
Integração da IoT: Temperature monitoring systems are becoming more integrated with IoT devices, allowing for real-time tracking and predictive maintenance during transportation.
Tendências -chave:
-
Biodegradable and Recyclable Materials: As eco-consciousness grows, many companies are shifting to environmentally friendly solutions.
-
Integration of AI for Temperature Control: AI can now help predict temperature fluctuations, optimizing dry ice usage for greater efficiency.
Common Questions About Dry Ice Bags and Vaccine Transport
P: Can dry ice bags maintain 2–8°C for long periods?
Sim, when used with proper insulation and PCMs, dry ice bags can maintain the 2–8°C range for up to 96 horas, depending on the quality of the packaging and monitoring systems.
P: How can I ensure that the vaccines stay safe during transport?
Monitor the temperature continuously using data loggers and ensure that the packaging is compliant with health regulations to maintain efficacy.
P: What are the risks associated with using dry ice in vaccine transport?
Enquanto o gelo seco é eficaz, improper use can lead to temperatures falling too low, potentially damaging vaccines. Adicionalmente, Gelo seco sublimata em dióxido de carbono, which can displace oxygen in enclosed spaces. Proper handling and ventilation are essential.
Conclusão
Dry ice bags can indeed help maintain vaccines within the critical 2–8°C temperature range when used properly. By pairing dry ice with high-quality insulated containers and PCMs, and employing temperature monitoring systems, it is possible to ensure vaccine efficacy during transport. With the continued advancement of cold chain technologies, the future of vaccine transport looks more secure and environmentally friendly.
Sobre Tempk
Tempk provides cutting-edge cold chain logistics solutions to ensure the safe and effective transport of vaccines and other temperature-sensitive products. Our products are designed to meet the highest standards, including FDA compliance, and provide reliable, sustainable transport options for the pharmaceutical industry. Contact us today to learn more about how our solutions can support your vaccine transport needs.