Approprié vaccine storage and cold chain practices are the backbone of immunization programs. Sans contrôle constant de la température, les vaccins perdent de leur efficacité, gaspiller de l’argent et mettre la santé publique en danger. Ce guide complet répond à vos questions sur le stockage des vaccins et la gestion de la chaîne du froid en 2025, des plages de température et des choix d'équipement à la surveillance, transports et innovations émergentes. Il reflète les dernières directives et tendances du marché et utilise un langage simple pour vous aider à protéger chaque dose..

Qu'est-ce qu'une chaîne du froid pour les vaccins et pourquoi est-elle essentielle? — comprendre comment un contrôle ininterrompu de la température préserve l'efficacité du vaccin.
Quelles températures sont sans danger pour les vaccins? — connaître les gammes pour les produits réfrigérés, vaccins congelés et ultra froids et pourquoi les écarts sont importants.
Comment choisir le matériel de stockage? — comparer les réfrigérateurs, congélateurs et unités ultra froides et découvrez pourquoi les réfrigérateurs de style dortoir sont une mauvaise idée.
Comment surveiller et enregistrer les températures? — découvrez le rôle des enregistreurs de données numériques (DDL), procédures opérationnelles standard et formation du personnel.
Quoi de neuf 2025? — explorez des innovations comme la blockchain, Capteurs IoT, solar powered units and portable cryogenic freezers that are transforming vaccine cold chain logistics.
What Is the Vaccine Cold Chain and Why Does It Matter?
The vaccine cold chain is the network of refrigerators, congélateurs, insulated containers and monitoring devices that keep vaccines within safe temperature limits from manufacture to administration. Maintaining this chain preserves potency; vaccines that are too warm can lose up to 20 % of their effectiveness in just an hour, while freezing aluminium containing vaccines causes clumping and irreversible damage. Health care professionals must manage inventory accurately, use reliable equipment and employ trained staff to ensure every dose remains effective. Poor cold chain management leads to waste—studies estimate as many as 35 % des vaccins sont compromis par une mauvaise gestion de la température—and risks outbreaks of vaccine preventable diseases.
Maintaining Potency: Plages de température recommandées
Vaccines are biological products that lose potency outside their prescribed ranges. Different categories require different conditions:
| Catégorie de vaccin | Plage de température | Exemples de vaccins | Importance |
| Réfrigéré | 2 °C–8°C (36 °F – 46 °F) | Influenza, DTaP, VPH, MMR and most routine vaccines | La gamme la plus courante; maintaining around 5 °C minimizes fluctuations. |
| Congelé | –50 °C – –15 °C (–58 °F – 5 °F) | Varicelle, mpox (Jynnéos), some COVID 19 formulations | Required for live attenuated vaccines; exposure to warmer temperatures can compromise viral components. |
| Très froid | –90 °C – –60 °C (–130 °F – –76 °F) | mRNA vaccines like Pfizer–BioNTech Comirnaty | Necessary for long term storage; some vials may be thawed and kept at 2 °C–8 °C for up to 10 semaines. |
These ranges must be respected at all times. Overheating degrades proteins and lipids, while freezing forms ice crystals that damage vaccine structure. Even a brief excursion above 8 °C may reduce potency by 20 %. When storing vaccines, keep them in their original boxes to protect from light and arrange by expiration date for “first expiring, first out” rotation.
Selecting the Right Vaccine Storage Equipment
Choosing appropriate storage units is critical. Purpose built, pharmaceutical grade refrigerators and freezers provide the most consistent temperatures. These units feature electronic thermostats, alarms and interior fans and are designed to maintain uniform temperatures even during frequent door openings. When pharmaceutical units are unavailable, standalone household refrigerators or freezers can be used, mais combination refrigerator/freezers are not recommended, and dorm style units with a single exterior door should never be used because they pose a significant risk of freezing vaccines. Each storage unit should have enough capacity to accommodate peak inventory without overcrowding; vaccines should be stored in the middle of shelves, away from walls and the door where temperature fluctuations are greatest. Avoid storing vaccines in vegetable bins or alongside staff lunches.
Practical Tips for Organising Vaccine Storage
Set thermostats at mid range: Adjust refrigerators to approximately 5 °C and freezers to around –25 ° C to minimize fluctuations.
Keep air circulating: Avoid overcrowding and place water bottles on shelves to help stabilise temperatures.
Étiqueter clairement: Designate shelves for refrigerated and frozen vaccines; do not store diluents or food in vaccine units.
Rotation des stocks: Store vaccines in original packaging, organise by expiry date and remove expired doses promptly.
Utiliser un équipement de secours: Maintain a back up refrigerator or freezer and ensure power supplies have surge protection or an uninterruptible power supply.
Cas du monde réel: Dans 2024, a clinic in New York avoided wasting more than $20 000 worth of vaccines when a freezer failed. Staff executed their emergency plan to transfer inventory to a calibrated backup unit that maintained 2 ° C - 8 ° C. This example underscores the value of preparedness and redundant storage.
Monitoring and Digital Data Loggers
Continuous temperature monitoring is the heart of vaccine cold chain management. The CDC recommends recording minimum and maximum temperatures at least twice daily and using enregistreurs de données numériques (DDL) to track temperatures continuously. Every storage unit should be equipped with a DDL that records temperatures at least every 30 minutes, has a buffered probe, out of range alarm, low battery indicator, display of current and min/max temperatures and an uncertainty of ±0.5 °C. Data should be downloaded and reviewed at least every two weeks or whenever an excursion occurs. Facilities must retain records for at least three years.
Why Digital Data Loggers Matter
Vaccines are fragile, and even small temperature deviations can render them ineffective. DDLs provide continuous monitoring that manual checks cannot match. Features to look for include:
High precision and calibration: Devices should offer ±0.1 °C accuracy and be calibrated at key temperatures such as 2 °C et 8 °C.
Sondes tamponnées: Glycol filled probes mimic the thermal response of vaccines, preventing false alarms caused by brief door openings.
Alertes en temps réel: Visual and audible alarms alert staff as soon as temperatures deviate.
Cloud connectivity: Remote access and cloud storage allow staff to review data, receive alerts and generate audit reports from anywhere.
Hot swappable calibration: Some devices, comme le 2025 EL USB VACX, offer on site calibration without downtime.
| DDL feature | Pourquoi ça compte | Avantage |
| Sonde tamponnée | Measures liquid temperature rather than air, reflétant la véritable température du vaccin | Avoids false alarms when doors open; prevents unnecessary transfers |
| Alarme hors de portée | Alerts staff immediately when temperatures deviate | Permet une action corrective rapide pour sauver les vaccins |
| Intervalle d'enregistrement programmable | Allows recording at least every 30 minutes | Provides detailed temperature history for audits |
| Glycol simulation | Simulates vaccine thermal response to minimize false alarms | Improves accuracy in real world conditions |
| Intégration cloud | Enables remote access, graphing and reporting | Simplifies audits and supports regulatory compliance |
Developing Standard Operating Procedures and Training
Monitoring alone is insufficient without clear procedures and trained personnel. Each facility should develop Standard Operating Procedures (Sops) covering routine storage and handling, surveillance de la température, interventions d'urgence et documentation. SOPs must be reviewed annually by a designated vaccine coordinator and updated whenever guidelines change. Staff should record min/max temperatures at the start of each workday and, if a device does not display min/max readings, check temperatures at least twice daily. La formation est essentielle: all staff handling vaccines should receive orientation and annual refresher courses. Scenario based emergency drills prepare teams to respond to power outages, equipment failures or natural disasters.
Transport and Distribution: Protecting Vaccines On the Move
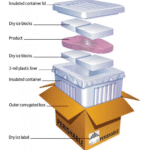
The cold chain extends beyond storage rooms. Vaccines must remain within their temperature ranges during transport from manufacturers to clinics. Logistics providers use conteneurs isolés, refrigerants and data loggers pour maintenir l’intégrité de la chaîne du froid. Selon une étude de marché, le mondial marché de la logistique de la chaîne du froid pour les vaccins était évalué à USD 3.5 milliards en 2024 et devrait atteindre USD 5.9 milliards 2034, grandir à un 5.3 % TCAC. Demand is driven by mRNA vaccines, advances in packaging technologies and growing awareness of health & wellness.
Choosing Packaging and Refrigerants
Different temperature ranges require different packaging methods. Dry ice provides temperatures around –78 °C and is suited for frozen or ultra cold shipments; gel packs and phase change materials maintain 2 °C–8 °C for refrigerated vaccines; liquid nitrogen enables cryogenic conditions below –150 °C for specialized cell therapies. The InsightAce report notes that packaging methods in the cold chain logistics market include glace carbonique, liquid nitrogen and gel packs. Use validated containers and pack out designs that match the required temperature range, and consider shipping durations and external conditions. Always include a calibrated DDL inside the shipment and choose shipping routes that minimize transit times.
IoT Sensors and AI Driven Logistics
Modern vaccine logistics increasingly rely on connected sensors and analytics. Capteurs intelligents compatibles IoT collect and share data on temperature, humidité et localisation en temps réel. Lorsque les capteurs détectent des niveaux de température dangereux, they automatically alert users through text, email or mobile apps. Many devices also provide GPS tracking, allowing visibility across the supply chain and enabling rapid intervention if deviations occur. Coupling IoT sensors with intelligence artificielle improves route planning; AI algorithms use real time traffic and weather data to optimize routes, reducing transit time and minimizing the risk of temperature excursions. Predictive analytics identify patterns and trigger alerts before excursions happen. These technologies strengthen supply chain resilience and help meet regulatory requirements.
Innovations Shaping the 2025 Vaccin Cold Chain
Beyond basic monitoring and transport, 2025 brings transformative technologies that enhance transparency, durabilité et efficacité:
Blockchain pour une traçabilité de bout en bout: Les grands livres distribués enregistrent chaque transaction dans la chaîne d'approvisionnement, creating a tamper proof log of temperature, location and hand off events. This transparency eliminates data manipulation and ensures regulatory compliance across stakeholders.
Chambre froide à énergie solaire: Off grid solar units provide reliable refrigeration in areas with unstable electricity. Dans 2024, l'électricité commerciale coûte en moyenne 13.10 centimes par kilowattheure, whereas solar rates ranged from 3.2 à 15.5 centimes par kWh. Solar cold storage reduces energy costs and extends cold chain coverage to rural areas.
Capteurs intelligents compatibles IoT: Wireless sensors offer real time temperature and location data, send alerts upon excursions and reduce operational risks. Combiné avec le GPS, they allow complete visibility from origin to destination.
Optimisation des itinéraires grâce à l'IA: Artificial intelligence analyses traffic and weather patterns to select routes that maintain temperature stability. Predictive analytics identify potential excursions before they happen.
Congélateurs cryogéniques portables: New compact freezers maintain temperatures as low as –80 °C to –150 °C, enabling the safe transport of cell and gene therapies and ultra cold vaccines. Integrated real time tracking and alerts ensure compliance.
Emballage durable: Les entreprises adoptent les contenants isothermes recyclables, enveloppes thermiques biodégradables et compresses froides réutilisables pour réduire l'impact environnemental. Sustainable solutions protect vaccines while aligning with corporate ESG commitments.
2025 Derniers développements et tendances
The cold chain industry is experiencing rapid growth and heightened regulatory scrutiny. Les principaux développements comprennent:
Accelerating market expansion: The global cold chain market is expected to grow from USD 418.81 milliards en 2025 à USD 1,416.67 milliards 2034, représentant un 14.5 % taux de croissance annuel composé. L’Amérique du Nord détient environ 36 % of revenue and continues to invest in energy efficient technologies.
Rising demand for mRNA and biologic vaccines: The ongoing rollout of mRNA boosters and cell therapies necessitates ultra cold storage and robust monitoring, stimuler les investissements dans les équipements cryogéniques.
Stricter compliance requirements: Regulatory bodies such as the CDC, WHO and EU GDP mandate continuous temperature monitoring, detailed record keeping and regular calibration. Facilities must demonstrate data integrity and readiness for audits.
Emergence of connected cold chains: Integration of IoT sensors, AI and blockchain fosters end to end visibility, enabling proactive intervention and improving patient safety.
Accent sur la durabilité: Governments and companies are prioritizing renewable energy and recyclable materials to reduce the environmental footprint of cold chain operations.
Insistance au marché: Growth and Investment Drivers
The cold chain sector supports both food and pharmaceutical industries, but vaccines represent a particularly high stakes segment. Selon la recherche prioritaire, le marché mondial de la chaîne du froid devrait croître à 14.5 % TCAC depuis 2025 à 2034. Entre-temps, the dedicated marché de la logistique de la chaîne du froid pour les vaccins—covering storage, packaging and transportation—will expand from USD 3.5 milliards en 2024 à USD 5.9 milliards 2034. Factors driving growth include:
Surging vaccine production: Demand for mRNA boosters, varicella and other live vaccines necessitates more cold chain capacity.
Innovations technologiques: Capteurs IoT, blockchain and AI improve efficiency and reduce waste.
Expanding healthcare access: Rural immunisation programs and global disease eradication initiatives require reliable cold storage in remote areas, spurring investment in solar powered and portable solutions.
Conformité réglementaire: Stricter guidelines for temperature monitoring, documentation and security compel facilities to upgrade equipment and systems.
Questions fréquemment posées
Q1: How long can mRNA vaccines be stored at refrigerator temperatures?
Après décongélation, some mRNA vaccines such as the Pfizer–BioNTech Comirnaty may be kept at 2 ° C - 8 ° C pour jusqu'à 10 semaines. Always check the manufacturer’s guidelines and monitor temperatures continuously.
Q2: What should I do if a vaccine is exposed to temperatures above 8 °C?
Any temperature excursion may degrade potency. Mettez immédiatement en quarantaine les vaccins concernés, label them “do not use,” and contact the manufacturer or immunisation program for guidance. Studies show that even a one hour exposure above 8 °C may reduce vaccine effectiveness by jusqu'à 20 %.
Q3: Can I store vaccines in a household refrigerator?
Household refrigerators may be used if pharmaceutical grade units are unavailable, mais combination units and dorm style fridges are not acceptable. Vaccines should be stored in the middle of shelves away from walls and the door.
Q4: How often should I record temperatures?
The CDC recommends checking and documenting minimum and maximum temperatures au moins deux fois par jour and downloading data from digital loggers every two weeks or after any excursion. If your logger displays min/max readings, record them at the start of each workday.
Q5: What should an emergency cold chain plan include?
A robust plan should cover backup power sources, alternative storage locations, transport containers and emergency contact information. Conduct regular drills and ensure all staff know the steps to transfer vaccines safely during power outages or equipment failure.
Résumé et recommandations
Principaux à retenir: Keeping vaccines potent in 2025 requires strict adherence to temperature ranges (2 °C–8 °C for most vaccines, –50 °C– –15 °C for live attenuated vaccines and –90 °C– –60 °C for mRNA formulations). Use purpose built storage units, avoid overcrowding and maintain clear labelling. Implement calibrated digital data loggers and record temperatures at least twice daily. Élaborer des SOP, train staff regularly and prepare for emergencies. Embrace innovations like IoT sensors, AI route optimisation and solar powered freezers to enhance efficiency and sustainability.
Conseils d'action: Start by auditing your current storage units and replacing any combination or dorm style refrigerators. Invest in high precision DDLs with glycol buffered probes and cloud connectivity. Write or update SOPs covering routine handling, procédures de surveillance et d’urgence, and schedule regular drills. Explore emerging technologies—IoT sensors, AI enabled route optimisation and portable cryogenic freezers—to future proof your cold chain. For tailored recommendations, consult cold chain specialists or contact us at Tempk.
À propos du tempk
Tempk is a leading innovator in cold chain solutions for healthcare and life sciences. We design and manufacture pharmaceutical grade refrigerators, congélateurs, insulated containers and state of the art temperature monitoring systems. Our products feature advanced insulation, digital data loggers with cloud connectivity and options for solar power integration. We are committed to sustainability and offer reusable packaging and biodegradable thermal wraps to reduce environmental impact. Avec un R dédié&D team and strict quality standards, we help you deliver vaccines safely and efficiently.
Besoin d'aide? Contact our specialists for a customised cold chain assessment and discover how Tempk’s solutions can safeguard your vaccines and support compliance.