Dry Ice Bag for Vaccines: How to Size, Pack & Comply
Last updated: September 18, 2025. This consolidated guide merges three expert drafts and aligns them to 2025 on‑page SEO and compliance standards.
In short: use a dry ice bag for vaccines only when the label or validated pack‑out requires frozen or ultra‑low temperatures, then size the dry ice for the lane’s worst hour and keep CO₂ venting and UN1845 labeling crystal‑clear.
-
How to size a dry ice bag for vaccines for 24–72 hours with lane risk in mind
-
How to pack, vent, and mark UN1845 under IATA PI 954 (air) and field SOPs
-
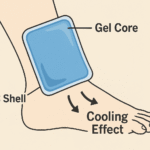
When to avoid dry ice and use 2–8 °C cool packs instead
-
How food lanes differ (a short dry ice bag for meat section) and what that means for you
-
What 2025 tech and policy trends change your playbook
How much dry ice should your dry ice bag for vaccines hold?
Short answer: For a typical validated shipper, plan 5–10 lb per 24 h, then round up and add ~20% for handoffs and hot ramps. Place the dry ice bag for vaccines above the payload (cold air sinks) and keep a standoff to avoid brittle contact. Start a logger and size to the lane’s longest credible hour, not the brochure estimate.
Why this works: Field rules of thumb and qualification data converge around ~5–10 lb per day for small/medium ULT shippers, with sublimation accelerating when packages are opened or exposed to heat. If your OQ report lists a sublimation rate, multiply it by transit hours and add a 1.2 safety factor. Avoid using a dry ice bag for vaccines for routine 2–8 °C products—conditioned cool packs are safer for those lines.
UN1845 & PI 954 for your dry ice bag for vaccines—what matters in 2025?
Your outer box must read “Dry Ice” or “Carbon dioxide, solid,” UN1845, and the net dry ice mass in kg, on two opposing sides. Packages must vent CO₂; never seal inner liners or the dry ice bag for vaccines gas‑tight. Airline/operator variations apply, so copy the exact net kg onto paperwork. Trained staff, open vents, and a visible label keep auditors and carriers aligned.
| Sizing Input vs. Control | Typical Range | Practical Control | What it means to you |
|---|---|---|---|
| Ambient extremes | −10 °C to +43 °C | Size for the hottest hour; use 20% margin | Prevents mid‑lane warmup |
| Sublimation baseline | 5–10 lb / 24 h | Use upper bound for >2 handoffs | Keeps buffer for quick‑open events |
| Label & venting | UN1845 + net kg; vent path | Bag secured but not air‑tight | Avoids pressure, carrier rejections |
Practical tips to run your dry ice bag for vaccines today
-
Hot‑lane rule: Add 20% dry ice for >2 handoffs or >32 °C peaks.
-
Logger placement: Put the logger near the thermal core, not buried in pellets.
-
Quick‑open SOP: Open <60 s in a ventilated zone; reseal and log time‑open.
-
Bag spec: Use −80 °C‑rated PE or co‑extruded film (100–150 µm) with clip/tie closure—contain pellets, don’t trap gas.
Real case: A depot moved 6,000 mRNA doses on a 48 h route with two hubs. With a dry ice bag for vaccines loaded to 24 lb and a validated ULT shipper, excursions dropped to zero and claims halved in one quarter.
How do you pack and label a dry ice bag for vaccines safely?
Core steps (auditor‑friendly): Pre‑stage at label temperature → confirm vents → load vials in secondary + logger → fill and secure the dry ice bag for vaccines → maintain standoff → close shipper → mark UN1845 + net kg on two sides → document lot/ID/time. This keeps CO₂ release, traceability, and label compliance tight while protecting vials from thermal shock.
Extra context: Dry ice becomes CO₂ gas, so ventilation is a safety and regulatory requirement. OSHA/NIOSH exposure limits and IATA PI 954 are the anchors used by auditors; training plus a one‑page bench card prevents sealing vents by mistake. For 2–8 °C lines, use conditioned packs instead of a dry ice bag for vaccines to avoid freeze damage.
Lane calculator for your dry ice bag for vaccines (use for quoting, then validate)
When should you avoid a dry ice bag for vaccines?
Skip it when labels specify 2–8 °C storage or when the IFU disallows sub‑zero transport. In those cases, use PQS‑aligned carriers and conditioned cool packs/PCMs. A dry ice bag for vaccines is reserved for frozen (−50 to −15 °C) or ultra‑low (−90 to −60 °C) shipments that your validation supports.
Mini self‑check for a dry ice bag for vaccines
-
Is your product labeled for frozen or ULT transport?
-
Is the package vented with UN1845 and net kg on two sides?
-
Did you plan ≥10 lb/24 h for typical shippers (plus 20%)?
-
Do pack‑out staff know CO₂ safety and “quick‑open” steps?
What changes when shipping food—dry ice bag for meat?
Food lanes share physics but differ in targets. A dry ice bag for meat keeps cuts hard‑frozen and dry; gel packs keep cooked or chilled foods at 2–8 °C. Use ≥2 in insulation for 24–48 h, position dry ice on top, and leave liners slightly open to vent gas. Label Class 9, UN1845, and net kg clearly. Plan shipments early in the week to avoid weekend holds.
2025 trends that change your dry ice bag for vaccines plan
-
Smarter shippers: Integrated loggers and CO₂ sensors with QR IFUs raise first‑attempt success.
-
Sustainability: Recovered‑carbon dry ice and recyclable liners reduce footprint without losing hold time.
-
Better planning models: Teams combine qualification loss rates with lane history to right‑size the dry ice bag for vaccines from day one.
[Tip: save or screenshot this section for training.]
FAQ: dry ice bag for vaccines
Can I use a dry ice bag for vaccines stored at 2–8 °C?
No. Use conditioned cool packs and PQS carriers; dry ice risks freezing these products.
How much dry ice per 24 h should I plan?
Use 5–10 lb per day for typical shippers, then add ~20% for handoffs and heat exposure.
What labels are mandatory for air shipments?
Mark “Dry Ice/Carbon dioxide, solid,” UN1845, and the net dry ice mass in kg on two opposing sides; ensure CO₂ can vent.
Is there an exposure risk when opening shippers?
Yes. Open in ventilated areas and train staff; CO₂ can displace oxygen quickly in closed rooms.
Sources aligned: CDC Vaccine Storage & Handling Toolkit (2024), WHO PQS cold chain guidance, IATA PI 954, FAA/ICAO dry ice rules, OSHA/NIOSH CO₂ exposure limits, USP <1079>.
Summary & recommendations for your dry ice bag for vaccines
Focus on four habits: use a dry ice bag for vaccines only when labels allow, size at ≥10 lb/24 h plus margin, keep packages vented and marked UN1845, and train teams on CO₂ safety and quick‑open SOPs. These moves cut excursions, reduce claims, and keep audits clean.
Next steps: Audit your lanes; validate a pack‑out with logging; standardize UN1845 labels; coach a 15‑minute bench drill; and revisit dry‑ice mass after two cycles.
About Tempk
We help biopharma and food teams move temperature‑sensitive products with audited reliability. Our validated shippers, dry ice bag for vaccines kits, and lane‑specific SOPs reduce excursions and documentation work. Customers report steadier dwell, faster acceptance, and fewer claims after standardizing our pack‑outs and calculators.
Talk to us: Book a 30‑minute consult to match a dry ice bag for vaccines to your riskiest lane—sizing, labeling, and training included.